 Simon Lam Sameer Gafoor Stefan Bertog Horst Sievert 德国法兰克福心血管中心
Simon Lam Sameer Gafoor Stefan Bertog Horst Sievert 德国法兰克福心血管中心
心房颤动(房颤)是一种常见疾病,发病率随年龄的增长而增加。非瓣膜性房颤的卒中年发病率为5%。血栓预防抗凝治疗仍以华法林为主,同时很多新型抗凝药物问市。但是,不论应用哪种抗凝药物,抗凝治疗都会面临出血风险。此外,还存在药物不耐受、依从性差、药物相互作用、需要绝对的饮食限制和定期监测INR等问题。这使得抗凝治疗药物每年的停药率高达38%。即使在那些应用华法林的患者中,也仅有40%的患者能达到治疗范围。对中国人群而言,中草药的应用易导致的与华法林的药物相互反应,停药现象更为严重。
左心耳封堵术治疗临床研究结果
越来越多的证据显示,左心耳封堵术是抗凝治疗一个很好的替代治疗方法。主要基于以下原因:①导致房颤卒中发生的栓子90%来源于左心耳;②外科左心耳结扎术具有很好的临床应用经验。左心耳长为2~4 cm,由一个或多个小叶构成。房颤时心脏收缩乏力,血流缓慢,易诱发血栓形成。PROTECT AF研究中,将CHADS2评分(评分项目包括充血性心力衰竭、高血压、年龄>75岁、糖尿病、卒中史或短暂性脑缺血发作)≥1的患者按2:1的比例随机分为WATCHMAN植入组或华法林组。随访1065患者-年的结果显示,WATCHMAN组的主要疗效事件发生率为3.0/100患者-年(95%CI:1.9~4.5);华法林组为4.9/100患者-年(95%CI:2.8~7.1)。与华法林组相比,WATCHMAN组发生主要疗效事件的RR为0.62,95%CI为0.35~1.25。干预非劣效性的可能性高于99.9%。主要疗效终点(无卒中、死亡及全身性栓塞)结果表明,与华法林治疗相比,左心耳封堵术具有非劣效性。
在PROTECT AF试验中,与华法林组相比,WATCHMAN组主要安全性事件发生更频繁(7.4/100患者-年 vs. 4.4/100患者-年,RR1.69,95%CI:1.01~3.19)。但对PROTECT AF试验早期及晚期阶段的亚组分析及其与CAP注册研究的对比发现,7天内手术/设备相关的不良事件从早期的10%降至晚期的5.5%,CAP时降至3.7%。三个阶段严重心包积液的发病率分别为6.3%、3.7%和2.2%,手术相关的卒中发病率分别为1.1%、0.7%和0%。这提示,随着术者经验增加,左心耳封堵术的安全性显著改善。
PREVAIL试验的试验设计与PROTECT AF试验相似。入选407例患者,以2:1的分配比例随机分为干预组与对照组。与PROTECT AF试验相比,尽管研究中有很多新的术者,但PREVAIL试验中患者的手术并发症显著减少。初步结果表明,与对照组的抗凝治疗相比,手术干预术后18个月的卒中及栓塞事件发生率并无劣效性。
左心耳封堵治疗最新进展
美国St Jude医疗器械公司生产的Amplatzer Cardiac Plug(ACP)是另一种左心耳封堵术常用封堵器。其应用的相关数据已经被公布。ACP上市后注册研究正对其进行评估。目前得到的研究结果令人鼓舞。目前欧洲已经有很好的ACP植入经验,亚太地区应用也越来越多。
采用LARIAT进行经皮左心耳缝扎,是左心耳封堵术的替代方法。近期发表的一项对89例患者的观察性研究显示,经食管超声心动图(TEE)随访一年时,98%的患者实现完全性结扎。这种手术方式拥有良好的疗效,并发症及围术期不良事件的发生率也较低。
总之,经导管左心耳封堵术是一种具有良好应用前景的传统抗凝治疗的替代方法,尤其是对存在抗凝治疗禁忌证的患者而言更是如此。目前我们所面临的问题是:其能否应用于不存在抗凝治疗禁忌证的患者?封堵器周边漏及左心耳闭合不全会对患者有何临床影响?我们应该如何将左心耳封堵术与新型抗凝剂进行对比分析?进一步的大规模随机试验有望帮助我们找到答案。
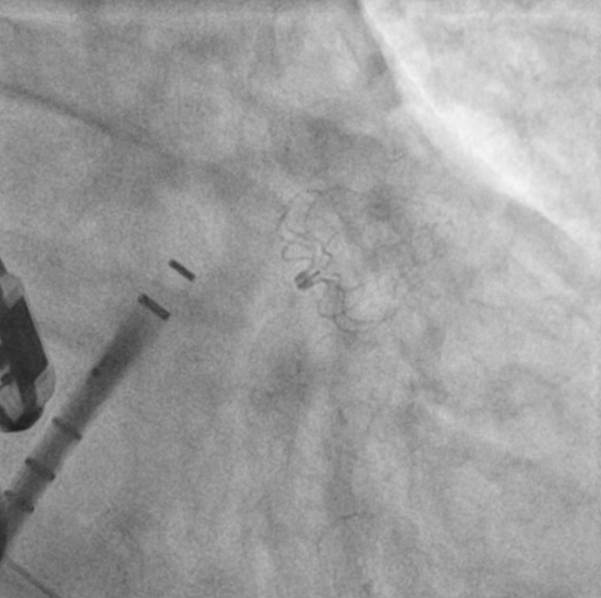
Figure 1 .Watchman Device post deployment in LAA
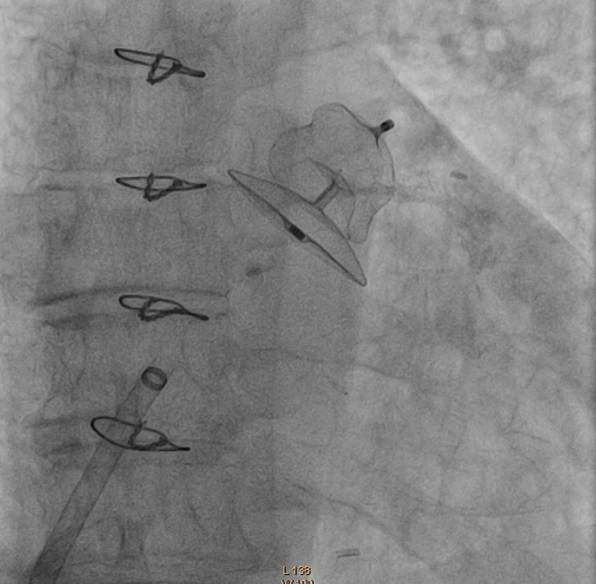
Figure 2. ACP Device post deployment in LAA
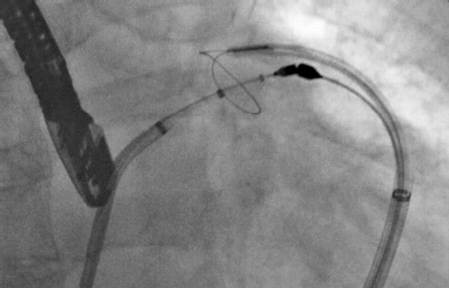
Figure 3. The LARIAT percutaneous LAA ligation system
Atrial fibrillation (AF) is a common condition with increasing incidence with age. The overall stroke rate in nonvalvular AF is 5% per year. Anticoagulation with warfarin has been the mainstay of treatment for thromboembolism prevention. There are also many novel anticoagulation agents coming into the market. However, the common concern is still bleeding risk, no matter which agent is used. In addition to that, there are problems with intolerance, poor compliance, drug interactions ,and the need for absolute dietary restrictions and regular INR monitoring. This is reflected by the high discontinuation rate of around 38% per year. Even in patients who can take warfarin, therapeutic range is only achieved in around 40% of patients. This may be even more of a problem in a Chinese population, in which herbal medicine is very popular, while herbal medicine has a lot of drug interactions with warfarin.
Clinical Data of LAA Closure
There has been emerging evidence showing catheter-based left atrial appendage (LAA) closure is a sound alternative. This concept is based on 2 insights: ① 90% of emboli in stroke patients with AF originate from left atrial appendage ;②experience from surgical LAA ligation. Left atrial appendage is an elongated structure of 2~4 cm with 1 or more lobules. Ineffective contraction in AF promotes stagnant blood flow and predispose thrombi formation. PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients with Atrial Fibrillation) was the landmark,multi-center trial with a non-inferiority design which randomized patients with CHADS2 Score≥1 (Congestive heart failure, hypertension, age>75, diabetes mellitus, prior stroke or transient ischaemic attack) into a WATCHMAN(Atritech, Boston Scientific, Natrick, MA, USA) implantation group and a warfarin therapy group in a 2:1 fashion.800 patients were enrolled. At 1065 patient-years of follow-up, the primary efficacy event rate was 3.0 per 100 patient-years (95% CI: 1.9~4.5) in the intervention group and 4.9 per 100 patient-years (95% CI: 2.8~7.1) in the control group (RR 0.62,95%CI:0.35~1.25). The probability of non-inferiority of the intervention was more than 99.9%.Based on the primary efficacy endpoint (freedom from stroke, death ,and systemic embolization), this study demonstrated non-inferiority of the LAA closure strategy as compared to warfarin treatment.
In the PROTECT AF Trial, primary safety events were more frequent in the intervention group than in the control group (7.4 per 100 patient-years vs.4.4 per 100 patient-years, RR 1.69; 95% CI:1.01~3.19). However, in sub-analysis between early phase of PROTECT AF Trial versus late phase, and also compared with the CAP (Continued Assess Protocol Registry), the incidence of procedure/device related adverse events (within 7 days) dropped from 10%, 5.5% to 3.7%. Incidence of serious pericardial effusion dropped from 6.3%, 3.7% to 2.2% and procedure related stroke from 1.1%, 0.7% to 0%. This reflects,as with all interventional procedures,that there is a significant improvement in the safety of left atrial appendage closure (in this series, WATCHMANDevice) with increased operator experience.
PREVAIL Trial had a similar design as PROTECT AF. 407 patients were randomized in a ratio of 2:1 (device versus control). Compared to PROTECT AF, patients in PREVAIL Trial had significantly less procedural complications, despite including new operators. Preliminary results suggested non-inferiority in terms of 18 month stroke and embolism rates.
New Advances in LAA Closure
Amplatzer Cardiac Plug (AGA, St Jude Medical, Minneapolis, MN, USA) is another commonly used LAA occluder with the most published data. This is now currently evaluated in the ACP Post Marketing Registry and interim results are also promising. There is a wide experience in ACP implantation in Europe with increasing number of implantations in the Asia Pacific region.
Percutaneous LAA suture ligation with LARIAT Device (SentreHEART, Redwood City, California) was an alternative approach for LAA occlusion. An observational study with 89 patients was recently published which showed complete ligation in 98% patients in 1 year TEE follow up andcan be performed effectively with acceptably low access complications andperiprocedural adverse events.
In conclusion, transcatheter LAA closure was a promising alternative to traditional anticoagulation therapy especially for patients who are contraindicated to anticoagulation therapy. The next questions are: Can this be applied to patients without contraindication to anticoagulation? What are the clinical impact of peri-device leak and incomplete LAA closure? How to compare LAA Occlusion with emerging new novel anticoagulation agents? - Further larger-scaled, randomized study might be able to provide the answer.
[下一页] [1] [2] [3]